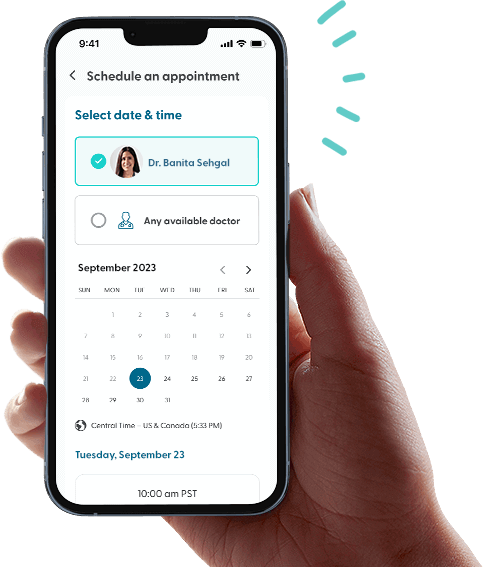
Talk to your doctor now.
Feel Better About Your Healthcare.
LifeMD provides easy online access to board-certified doctors who care about your health.
Looking for a primary care provider?
Need to speak to a doctor now?
Looking to try GLP-1 medications?

Weight Management Program
Unlock your full weight loss potential with expert care and access to medications like Zepbound™, Wegovy®, Ozempic®, Mounjaro®, and Saxenda®.
Special Offer: Save up to 40% Off

Virtual Primary Care
Keeping on top of your health and well-being can be a grind. That’s not the case at LifeMD. Patients are our number one priority, which is why we are available when you need care. Not the other way around.
Special Offer: Start Today for $39/month!
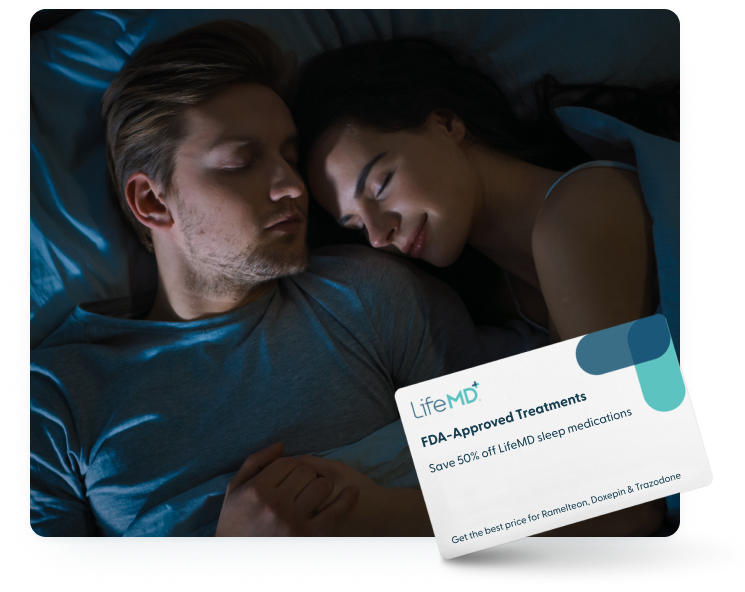
Prescription Sleep Medications
As the world’s leading online healthcare provider, LifeMD has the ability to prescribe safe, nonaddictive prescription sleep medications that can help you finally get to bed at night so you can conquer the day.
Special Offer: Save 50% Off + Free Consultation
Dedicated to providing the care you deserve.
LifeMD takes the system out of healthcare.
Easily access the best care
Doctor visits whenever and wherever you need it.
-
Book an appointment
Schedule a visit with a board-certified doctor at a time that works best for you.
-
Get the answers you need
Receive quality care, from diagnosis and prescriptions to lab orders and more.
-
Enjoy 24/7 peace of mind
Rest easy knowing that LifeMD is here for all your primary, urgent, and chronic health needs.
 Save 90%
Off Labs & Prescriptions
Save 90%
Off Labs & Prescriptions

Meet your LifeMD medical care team
Learn about the medical experts who inspire better healthcare.
Dr. Gupta
Allergies, Immunology, Pediatrics
Dr. Gupta is triple board-certified and holds certifications from the American Board of Allergy and Immunology, the American Board of Internal Medicine, and the American Board of Pediatrics.
Book AppointmentDr. Sehgal
Internal Medicine
Dr. Sehgal is board certified in internal medicine and has been practicing medicine for over 22 years. Before transitioning to telehealth, she worked mainly with a multi-specialty healthcare group, providing individualized care to over 2,200 patients.
Book AppointmentDr. Guirguis
Internal Medicine
Dr. Guirguis earned his D.O. from Nova Southeastern University, College of Osteopathic Medicine. He’s board-certified in Internal Medicine and has a special interest in preventive medicine and nutrition.
Book AppointmentDr. Moduthagam
Family Medicine
Dr. Moduthagam is a board certified physician in Family Medicine. She received a B.S. degree in Biochemistry from the University of Illinois in Chicago, Illinois before pursuing her medical studies. She is dedicated to thoughtful, insightful patient care.
Book AppointmentDr. Culpepper
Family Medicine
Dr. Culpepper has been practicing General Internal Medicine for over 30 years and is guided by his passion to help his patients live healthier lives. He is board-certified in Internal Medicine and also holds a professional degree in pharmacy.
Book AppointmentDina Whiteaker, APRN
Family Medicine
Dina earned her BSN from Methodist College in Omaha. She later graduated from the University of Nebraska Medical Center with an MSN and went on to become a family nurse practitioner. Dina has been working with patients for 12 years.
Book AppointmentDr. Puopolo
Family Medicine
Dr. Puopolo earned his M.D. from the Boston University School of Medicine. He later completed a combined Family Medicine and Psychiatry residency program in the U.S. Army at Tripler Army Medical Center in Hawaii. Dr. Puopolo has been practicing medicine for 22 years.
Book AppointmentLaurenmarie Cormier, NP
Family Medicine
Laurenmarie began her career as an ER nurse at a level 1 trauma center before transitioning to telemedicine. With a deep foundation in this work, she supports programs that improve patient access to timely and effective care.
Book AppointmentHarmony Vance, APRN
Family Medicine
Harmony has been caring for patients for more than 20 years in various roles in the medical field. In 2018, she graduated with a Master’s of Science Degree with a specialization in Family Nursing.
Book Appointment
Dr. Gupta
Allergies, Immunology, Pediatrics“ Striving to improve and give you the best treatments.
BIO:
Dr. Gupta is triple board-certified and holds certifications from the American Board of Allergy and Immunology, the American Board of Internal Medicine, and the American Board of Pediatrics.
Reviews:
“Dr. Gupta was amazing. Would recommend Dr Gupta and the service highly.”
“Very knowledgable. Personable. Made me feel comfortable about getting in control of my health when I was feeling embarrassed about neglecting it for some time.”
“Even throughout my busy day, I’m able to go over my medical needs and concerns with Dr. Gupta and she’s always outstanding! Thank you so much!”
“Very nice, straightforward, and thorough. Surpassed my expectations for my first virtual health appointment.”
Names redacted to protect patient privacy.

Dr. Sehgal
Internal Medicine“ My care philosophy is treating every patient with empathy and compassion.
BIO:
Dr. Sehgal is board certified in internal medicine and has been practicing medicine for over 22 years. Before transitioning to telehealth, she worked mainly with a multi-specialty healthcare group, providing individualized care to over 2,200 patients.
Reviews:
“I truly appreciated how knowledgeable my doctor was regarding COVID. She gave me direct, informative information and allowed me to participate in my care. Thank you!”
“The doctor explained everything in detail, was very kind and made me feel she is in this with me and will help me get healthy as long as I put in effort as well. Time is of the essence and I need to get well ❤️🩹”
“Dr. Sehgal always listens and explains very thoroughly. Very satisfied!”
“Dr. Sehgal is amazing. Super responsive, listens to my concerns and is always straight forward and informative. Treatment plans always take into consideration what is best for my whole being and best self. I want her as my doctor for life! ”
Names redacted to protect patient privacy.

Dr. Guirguis
Internal Medicine“ I prioritize your well-being and work together to achieve your healthcare goals.
BIO:
Dr. Guirguis earned his D.O. from Nova Southeastern University, College of Osteopathic Medicine. He’s board-certified in Internal Medicine and has a special interest in preventive medicine and nutrition.
Reviews:
“My experience with Dr. Guirguis was great. He helped me with all my questions and concerns.”
“Very understanding and thorough with going through my medical history. He addressed my concerns and was very helpful. Thank you!”
“Thorough Doctor. Analyzed my risk factors and provided covid treatment without a 4hr wait at a local urgent care.”
“Listened to all my symptoms and prescribed what I needed. Easy and swift way to solve my health issue.”
Names redacted to protect patient privacy.

Dr. Moduthagam
Family Medicine“ I strive to deliver the highest quality of care to all of my patients.
BIO:
Dr. Moduthagam is a board certified physician in Family Medicine. She received a B.S. degree in Biochemistry from the University of Illinois in Chicago, Illinois before pursuing her medical studies. She is dedicated to thoughtful, insightful patient care.
Reviews:
“Can't express enough how grateful I am for her and this tele-health service. I'd give 6 stars if I could for Dr. Moduthagam!”
“Very polite and informative. Wants to make sure the patient has plenty of open to floor to discuss how they feel and take their opinions into account. Great experience!”
“I did not know what to expect as a first time telemedicine user. The process was simple and I felt like the Dr took ample time to get to know me and my symptoms. I will definitely use again and recommend to others!”
“She was so incredibly kind and she made me feel so heard! Cannot thank her for this experience enough!”
Names redacted to protect patient privacy.

Dr. Culpepper
Family Medicine“ I’m always looking for ways to improve my service and patient outcomes.
BIO:
Dr. Culpepper has been practicing General Internal Medicine for over 30 years and is guided by his passion to help his patients live healthier lives. He is board-certified in Internal Medicine and also holds a professional degree in pharmacy.
Reviews:
“"The most incredible doctor I have ever been associated with. Very understanding and knowledgeable! Incredible appointment thank you so much!”
“Simply the best PCP I’ve had in my adult life.”
“Wonderful experience. Dr. Culpepper was amazing in helping me through my tough situation.”
“Very easy, pleasurable experience with both the pre-call and call with the doctor. Asked great questions, cared about my health, listened and conversation was just as easy as if in an office.”
Names redacted to protect patient privacy.

Nicole Baldwin, APRN
Family Medicine“ I take a patient-centered approach to healthcare, where my patients are always my top priority.
BIO:
Nicole is a board-certified, multi-state nurse practitioner specializing in Urgent Care, Primary Care, Women’s Health, Men’s Health, Pediatrics, and Orthopedic Trauma.
Reviews:
“Nicole was fabulous and kind and so understanding. I could not be more pleased with the help she has given me.”
“Kind, friendly, very helpful clear and concise, straight to the root of the problem and offering assistance.”
“Very helpful, informative, was able to help me when I needed it will definitely recommend.”
“She is wonderful! Doesn’t give the “awkward” doctor feeling you get going to any doctor. Very personable and friendly.”
Names redacted to protect patient privacy.

Jackie Rosenhein, APRN
Family Medicine“ I believe that healthcare is a partnership between my patient and me, where we work together to achieve optimal health.
BIO:
Jackie has worked in healthcare for 30+ years and has been a nurse practitioner for the last five. She loves being able to help her patients through shared decision-making so they can reach their health goals.
Reviews:
“On vacation in Florida caught a cold!! Uugghh!! Jackie Ramsey-Rosenhein saved me from losing out on a wonderful experience!!”
“Amazing. I absolutely loved her. She listened to me and answered all my questions. I definitely recommend her.”
“Easy to schedule, on time, took care of my needs. Love not having to drive to the doctors office, sit through long waits… etc.”
“Professional, friendly and patient. Wonderful experience. So grateful for this service, as my own physicians office told me to call back Monday.”
Names redacted to protect patient privacy.

Dina Whiteaker, APRN
Family Medicine“ I’m dedicated to providing my patients with personalized and comprehensive care.
BIO:
Dina earned her BSN from Methodist College in Omaha. She later graduated from the University of Nebraska Medical Center with an MSN and went on to become a family nurse practitioner. Dina has been working with patients for 12 years.
Reviews:
“Dina took into account my pre existing conditions and decided on the best way to treat my flu symptoms, excellent provider who really cares.”
“Amazing experience and very helpful in getting medicine needed to feel better without sitting ins doctors office with extreme wait times.”
“Dina was very knowledgeable and thorough. Even though it is telehealth you can tell she really cares and wants you to get better. Lovely experience.”
“Wonderful and kind. Really appreciated the experience. Will definitely return for future needs. Dina is a caring provider. Very professional!”
Names redacted to protect patient privacy.

Dr. Puopolo
Family Medicine“ I believe in treating the whole person, not just their medical condition.
BIO:
Dr. Puopolo earned his M.D. from the Boston University School of Medicine. He later completed a combined Family Medicine and Psychiatry residency program in the U.S. Army at Tripler Army Medical Center in Hawaii. Dr. Puopolo has been practicing medicine for 22 years.
Reviews:
“Thorough review of my current health. Guidance was given to continue to see improvements in the future. Feedback was great!”
“Friendly, helpful, and fast. Quickly addressed an urgent concern over Thanksgiving week when I couldn’t get into my primary care doctor for over a month. Much preferred over the normal medical process too. Thanks!”
“Patient listener, very understanding, attended all doubts and gave me peace of mind. Thank you Dr Anthony. Appreciate your time & advice.”
Names redacted to protect patient privacy.

Laurenmarie Cormier, NP
Family Medicine“ I value open communication and encourage my patients to share their concerns and questions.
BIO:
Laurenmarie began her career as an ER nurse at a level 1 trauma center before transitioning to telemedicine. She has extensive experience in building telehealth programs to better serve diverse patient populations. With a deep foundation in this work, she supports programs that improve patient access to timely and effective care.
Reviews:
“Very professional, kind, knowledgeable and listened to needs and concerns ty Laurenmarie!”
“She was great, very informative, helpful, friendly, asked good questions, helped me make a good decision”
“She is great! Takes care of what I need quickly and efficiently and is very easy to talk to.”
Names redacted to protect patient privacy.

Harmony Vance, APRN
Family Medicine“ I’m here to support my patients every step of the way, I’m committed to their long-term health and well-being.
BIO:
Harmony has been caring for patients for more than 20 years in various roles in the medical field. In 2018, she graduated with a Master’s of Science Degree with a specialization in Family Nursing. Harmony has spent the past few years working in telemedicine, Urgent Care, and Primary Care.
Reviews:
“Harmony provided professional care with compassion. I work in healthcare myself and have never received better care than I did with her. Many thanks for the help to lead me down the road to recovery.”
“Wonderful service! Fast diagnosis and prescription called in. Made appointment at 11:00 pm for my daughter at 6:40 am the next day. Super easy, fast and efficient!”
“This was the best doctors visit I have ever experienced in my life. Harmony was beyond helpful and pleasant.”
“It is clear that she genuinely is here to help people and cares about patients getting better. After experiencing discomfort for over a year, I am so grateful I found someone who is dedicated to helping me.”
Names redacted to protect patient privacy.
Verified Reviews
See why real patients choose LifeMD.
“Very kind, compassionate, and concerned with my wellbeing. I feel like I was listened to and taken seriously and given a few different options which made me feel in control of my health plan. ”
Brittany from California
Verified Patient
“Great virtual appointment for pediatric case of pink eye! The doc was easy to talk to and listened to what we had to say and made the whole process much easier.”
Shiloh from California
Verified Patient
“Dr. Puopolo is Fantastic! He has a great bedside manner, and he's likely the doctor you've been searching for. This service is very efficient and reliable, and you feel like you're in the doctors office receiving care, but it's from your own home. I will use this service again.”
Julie from Arizona
Verified Patient
“Awesome appointment quick and to the point. He was knowledgeable and helped with any questions that I had”
Kenton from North Carolina
Verified Patient
“Super intelligent, kind, and helpful. I was so scared and stressed prior to the call but Dr. Sehgal made sure I was heard, and my concerns were addressed, and that I accomplished my goal for this appointment. I would definitely see her again!”
Briana from North Carolina
Verified Patient
“I did not know what to expect as a first time telehealth user. The process was simple and I felt like the Doctor took ample time to get to know me and my symptoms. I will definitely use again and recommend to others!”
Michele from Texas
Verified Patient
“Very knowledgeable, kind, straight to the point, and very helpful. A delight to deal with. My first time using LifeMD and I am very pleased with my experience!”
Patrick from Montana
Verified Patient
“I had an AWESOME experience and I am so glad I found this site! My doctor was detailed and thorough. Medicine of the future is here!”
Tamara from Alabama
Verified Patient
“She is incredible! Extremely helpful and genuinely cares for her patients. I feel very lucky to have her as my primary.”
Matisen from Florida
Verified Patient
“Easy to schedule, on time, took care of my needs. Love not having to drive to the doctors office, sit through long waits, etc.”
Lisa from Ohio
Verified Patient
“Great personal attention! Dr. Puopolo stopped and took time to actually listen to my concerns and questions.”
Roysbel from Florida
Verified Patient
“Incredible. Like years ago, where you can have that one on one relationship with your dr.”
Mari from Minnesota
Verified Patient
“Dr Asunta was fabulous. She was kind and thorough. Better than an in person visit.”
Julie from Connecticut
Verified Patient
“The Doctor was friendly with a very good bedside manner. He was very thorough in questioning my medical history, and current symptoms. We ended the appointment with a plan and course of treatment. I am very satisfied.”
Ingrid from Illinois
Verified Patient
“SHE IS AMAZING! Super easy to speak with and she just brightens your day. You almost forget you’re sick. ”
Maria from Florida
Verified Patient
“Awesome charismatic doctor who gave thorough explanations and a few home remedies along with sending me a prescription to my nearest pharmacy. Greatly appreciated! Healthcare is tough these days so thank you for still being so kind.”
Jaliyah from Georgia
Verified Patient
“She was wonderful! Such an easy experience. Highly recommend!”
Juliet from California
Verified Patient
“Such an amazing doctor. I had a wonderful appointment and it wasn't rushed, listened to my issues and gave me great options.”
Hunter from Florida
Verified Patient
“Dr. Sehgal is very professional yet approachable. I immediately trusted her and her advice.”
Laurie from California
Verified Patient
“Dr. Culpepper has an incredible bedside manner and really made me feel comfortable expressing my medical concerns. 10/10.”
Chanel from Texas
Verified Patient
4.9 Stars
Verified Average
Patient Reviews
Patient Reviews
Over 725K
Men & Women Have
Chosen LifeMD
Chosen LifeMD
1 Million
Online Completed
Consultations
Consultations









Skip the waiting room and meet your doctor today.
BECOME A MEMBERFeatured Articles & News
Exercise and Lifestyle
Ozempic, Wegovy, and Mounjaro: What You Need to Know About Weight Loss Drugs
Ozempic, Wegovy, and Mounjaro are experiencing a surge in popularity due to their success in helping people lose weight and keep it off for the long term. Learn the differences between the three medications, and find out which one may be right for you.
Lifestyle and Wellbeing
How to Successfully Overcome a Weight-Loss Plateau
Weight loss can be a challenging journey for many. It becomes especially difficult when...
Urinary Tract Infection (UTI)
Recognizing and Addressing Urinary Tract Infection Symptoms
Urinary tract infections (UTIs) are common infections that can affect both men and women...
Exercise and Lifestyle
A Comprehensive Overview of Menopause and Weight Gain
Menopause is a natural process that develops when women reach the end of their fertility...
Anxiety
9 Ways to Overcome Social Anxiety
Social anxiety disorder is a prevalent mental health condition that affects over 15 million Americans in their lifetime...
Feel better with LifeMD.
Your doctor is online and ready to see you.
Join LifeMD today and experience amazing healthcare, discounted labs and prescription medications... plus around-the-clock access to medical guidance.