LifeMD
Your personalized weight loss plan is now active.

10:05 AM
Achieve your weight-loss goals with GLP-1 treatments like Wegovy® and Zepbound®, guided by licensed providers every step of the way.
Personalized care for women, with HRT and lifestyle support to ease menopause symptoms and restore balance.
Simple, supportive mental health care on your terms, including access to prescription medication when appropriate.
Care without the wait—connect 24/7 with licensed providers for same-day prescription refills and common concerns like colds, flu, rashes, and more.

Weight Loss Money Back Guarantee
Lose 10% of your weight or your money back+

Lose weight with access to GLP-1 treatment options, clinical oversight, metabolic testing, and
ongoing support from licensed healthcare providers through LifeMD.
Lose weight with access to GLP-1 treatment options, clinical oversight, metabolic testing & ongoing support from licensed healthcare providers through LifeMD.









Prescription GLP-1 medications mimic natural hormones to regulate digestion and appetite. This makes them highly effective at helping patients lose weight and improve their health. Important Safety Information.
Take precaution with, and read all warnings concerning, GLP-1s, as they may cause serious side effects, including a risk of thyroid c-cell tumors. Do not use if you or your family have a history of a type of thyroid cancer called MTC (medullary thyroid carcinoma) or MEN 2 (multiple endocrine neoplasia syndrome).
“Since joining the LifeMD family, I have not only lost weight but changed the way I look at food. Making these changes has saved my life! To see my waist getting smaller is amazing. I absolutely hated trying on clothes because I didn’t feel pretty, but now I LOVE it!”
Alisa L.
Verified Patient

“I'm back to my pre-baby weight! My clothes look good on me. My relationship has improved because I feel more confident about myself. I am in a much better place with my mental health.”
Amanda B.
Verified Patient

“I had to get a whole new wardrobe. I have more energy now than I have for many years. My sleep has improved. My mood is boosted and I have the confidence to do anything I set my mind to. I am turning 60 this year and I feel like I am 40!”
Brenda J.
Verified Patient

“I have noticed a dramatic increase in my energy levels and have become a much happier person. I have also been able to fit into clothes I’ve had hidden in the back of my closet for years.”
Brian W.
Verified Patient

“My weight loss journey with LifeMD has been more than just a physical transformation—it’s been a journey of reclaiming my health and well-being. I feel more empowered, confident, and optimistic about my future.”
Bridgette R.
Verified Patient

“Life changing for our whole family and I want to share this experience with anyone that is looking to lose weight. My clothing is too big, my face looks completely different, I can cross my legs, I don’t feel so tired!!!”
Gabriela H.
Verified Patient

“I have gone from XL down to a medium! I am wearing clothes that I have not been able to wear in 2+ years. I was beginning to think that I would never fit in them again. I have more energy, and I even sleep better!”
Julie S.
Verified Patient

“My self-confidence is at an all-time high. Since starting my LifeMD weight loss journey, I have experienced zero negative mental side effects. Diets and appetite suppressants always left me feeling tired and constantly hungry.”
Josh B.
Verified Patient

“With so much weight lose I had to buy new clothes and couldn’t believe that I went from a size 14 to a 4. I never thought I could be a 4 in pants or buy an extra small in yoga pants. I love my body!”
Charity
Verified Patient

“I feel better in my body, able to wear the clothes I like, confidence improvement, and engaging more with people instead of isolating myself.”
Debby
Verified Patient

“I felt the same hopelessness and helplessness and seriously considered giving up, so I know how what that feels like and looks like. However, LifeMD is different. I strongly encourage you or anyone to just make the call...”
Valencia
Verified Patient

“The LifeMD Weight Management Program is different than other programs because they are there for you. They truly want you to succeed. My practitioner congratulates me on my success and has been transparent every step of the way.”
Lauren S.
Verified Patient

“I have remained at my target weight for almost a year now. Once you have gone through the process, you refuse to go back to the old you. Another note... nothing fits anymore and you have to buy an all-new wardrobe.”
Lewis F.
Verified Patient
“My clothes fit better, I have less pain in my joints, I get compliments from family and friends, I have more energy and a greater desire to do things, my confidence is sky-high, and I have no shame being with a group of people.”
Madden
Verified Patient

“If someone has given up on losing weight, I would tell them to definitely use this program — it will work. I have an entire new wardrobe! I have the energy to accomplish many things in a day and my self-confidence is at an all-time high.”
Mike S.
Verified Patient

“I can actually shop in my closet, wearing the clothes I didn’t want to get rid of ‘when I eventually lost the weight,’ but certainly couldn’t fit into. I am in an overall better mood as a result as well, because I am no longer self-conscious of how I physically look.”
Angie S.
Verified Patient

“I don't have to shop in the plus-size section anymore, I love how I feel in my clothes. My blood pressure has gone down as well as the swelling in my feet.”
Kristy P.
Verified Patient

“My doctor is great. He’s helped me understand things very easily. I check in with him once every month or two. It’s a nice phone call, we get through it really quick, and I’ve had nothing but success.”
Mike H.
Verified Patient

“I’m proud of myself. I was on blood pressure medication and I quit taking that. My pants size went from a very tight 38 to an easy 34.”
Gregg S.
Verified Patient

“I always tell people who are interested that it’s worth it. It’s nice when you can look in the mirror and say, ‘this is the body I’ve always wanted.’”
Kera K.
Verified Patient

“My clothes are fitting the way they should, and I’m losing the extra fluff around the muscle. I just feel way more comfortable in myself.”
Tara O.
Verified Patient

“My provider is great. She told me what I could expect and what I couldn’t expect. She gave me all the details of what I needed to know and was very encouraging. I like how she explained everything. She was very easy to talk to.”
Ismael F.
Verified Patient

“I’ve already dropped 6% of my body fat. Overall, my arms, my bust, my tummy, my waist, my hips… everything has gone down by five inches.”
Zippy A.
Verified Patient

LifeMD Weight Management Patient. Paid testimonial.
The LifeMD Weight Management Program includes access to medications like Wegovy® and Zepbound®, if appropriate.





Discuss your weight loss goals and health history with a LifeMD-affiliated provider through our confidential online questionnaire.
Your LifeMD-affiliated provider will review your information and work with you to create a treatment plan that can help you reach your target weight.
The LifeMD care team will check which medications are covered by your insurance and what your copay would be.
If you don’t have insurance or GLP-1 medication is not covered by your insurance, the team can present different options, including prescription discount “coupons” that can lower the cost of paying out of pocket.
LifeMD and partners will send your prescription to your local pharmacy. If your pharmacy doesn't have your medication, we can explore other options that can be mailed directly to your door.
From managing your medication to adopting healthy lifestyle changes, LifeMD will support you through every step of your weight loss journey.

Introducing the ultimate weight loss solution with access to medications and expert care.
Get Started Today Get Started Today
We’re here to help you achieve your weight loss goals with clinical guidance from licensed providers.

Clinical oversight, metabolic testing and ongoing support to help you lose weight and feel healthier overall.
This program combines groundbreaking GLP-1 medications, lab testing, and the knowledge of leading clinicians to create a highly effective approach to weight loss. We'll take your unique metabolic profile and lifestyle habits into account to help ensure you lose that extra weight - one of the most important things you'll ever do for your health.
We're so confident you'll succeed, that if you participate in our Weight Management Program and don't lose at least 10% of your body weight by the end of a year, you'll be eligible for a refund.
Licensed medical professionals provide comprehensive care, including your initial visit, coaching provided by your weight loss team and ongoing medical check-ins from the comfort of your home. Lab work may be required.
Begin your journey to a healthier lifestyle with our exceptional offer: Our program starts as low as $75, with the flexibility to cancel anytime. Our comprehensive program includes expert clinician consultations, personalized prescriptions, dedicated coaching, and essential lab work (if you haven't completed the required tests within the last 12 months). Medication cost is not included and varies based on insurance coverage or self-pay options.
Access to GLP-1 medications: As part of our commitment to offering cutting-edge treatment options, our program includes access to medications like Wegovy® or Zepbound®. Medication cost is not included and varies based on insurance coverage or self-pay options.
Insurance coverage assistance: Navigating insurance can be complex, which is why our support team helps you determine if your insurance covers GLP-1 medication.
Metabolic testing: Unlock the secrets to your body's unique metabolism with our comprehensive metabolic testing, which will help us create a tailored plan that works best for you.
Ongoing provider care: Expert medical providers will monitor your progress, make adjustments to your treatment plan as needed, and provide continuous care to ensure your long-term success.
GLP-Is are groundbreaking medications that have shown remarkable results in clinical studies. GLP-1 medications help regulate and improve the body's weight loss factors - such as blood sugar response and insulin. By changing these factors, the gut will send signals to the brain to improve metabolic function and regulate digestion and appetite, helping patients feel fuller, longer. In studies, patients lost up to 15-20% of their body weight* using branded GLP-1 medication.
In clinical studies, patients lost up to 15-20% of their body weight* using branded GLP-1s. Our easy-to-use Al tool provides you with a personalized estimate based on your current weight. See how much weight you can lose!
After you complete your online visit, a LifeMD-affiliated healthcare provider will review your answers and determine whether treatment is right for you. If approved, lab testing follows. Then, your medical provider will review your lab results and discuss a personalized treatment plan with you.
Insurance services for the cost of medications are provided through the LifeMD GLP-1 weight loss programs. Our partners will work directly with your insurance provider to help with the process of determining coverage for your GLP-1 medication, which is paid for separately from the LifeMD programs.
LifeMD does not accept insurance for its GLP-1 weight loss programs, but your insurance may help cover the cost of medication. At this time, those with any form of government healthcare coverage (including programs such as Medicare, Medicaid or TRICARE), whether primary or secondary, or government-related coverage such as Medicare Supplement Plans, are not eligible for LifeMD GLP-1 weight loss programs, as these plans typically do not cover the type of medication LifeMD programs may prescribe.
What if my insurance doesn’t cover the medication?
If for some reason your insurance will not cover the cost of your medication, you'll have the option to either pay for the medication out of pocket or cancel your LifeMD GLP-1 weight loss program membership. Given the cost of initial diagnostics, ongoing provider support, and insurance assistance, we are unable to offer refunds for previously incurred monthly membership fees.
Triple Therapy is a doctor-trusted treatment plan that consists of three medications (Metformin, Bupropion, and Topiramate) combined in carefully selected doses, with the aim of supporting your weight loss goals.
The Triple Therapy program is intended for patients who do not meet the BMI requirements to start a GLP-1 program but still seek help managing their weight. The BMI threshold for GLP-1s without a comorbidity is 30. Anyone with a BMI of 25 or greater may be eligible for the Triple Therapy medication pack. Triple Therapy is an all-inclusive price of $129/month.
Take the prescribed pills each day as directed by your LifeMD-affiliated healthcare provider. For the first 30 days, most patients are instructed to take their three pills, once a day, with or without food. After 30 days, many patients are instructed to take three pills twice a day (a total of six pills in total). Your provider will provide more specific instructions on when and how to use your treatment.
There are many benefits to using Triple Therapy as part of your weight loss journey. Here are three of the numerous reasons patients like this treatment:
It’s an oral treatment. No need for needles, wipes, or additional supplies. Simply take the prescribed pills each day — and you're set!
It’s cost-effective. A Triple Therapy subscription — which includes the cost of medication — is typically significantly less expensive than monthly costs associated with GLP-1 therapy, helping you save money while managing your health.
It’s accessible. Patients who don’t medically qualify for prescription GLP-1 medications like Wegovy may still qualify for Triple Therapy.
First, complete a free, online medical intake form. Then, you can join the LifeMD Weight Management Program (if you are a candidate) and schedule an appointment with a LifeMD-affiliated healthcare provider. If appropriate, they will prescribe treatment. Note: Labs are encouraged, but not required before you are initially prescribed treatment.
LifeMD Weight Management Patient. Paid testimonial.
Losing weight is more affordable than ever.
Are you ready to lose weight and improve your health? LifeMD offers the resources you need to reach your goals — all for an affordable monthly price.
Join LifeMD Today Join LifeMD Today
See why real patients choose LifeMD.
I’ve tried everything and nothing’s really worked but this. My confidence has improved 100%. This is the body I’ve always wanted.
Verified Patient
Kera K.

I think LifeMD’s Weight Management Program is probably the easiest thing I’ve ever done. I’ve lost 20 pounds already. Every day, I can’t wait to get out of bed. I feel amazing.
Verified Patient
Mike H.

As of last week, I was down 10 pounds in five weeks. Everybody says, ‘It’s not the number on the scale that matters,’ but to me, it does. It really helps your confidence.
Verified Patient
Susan J.

Before, I’d always be thinking about my next meal and I would crave sweets at night. It was like an addiction. But now, my goodness, I feel like I’ve been freed. It’s like my brain’s been rewired.
Verified Patient
Mary H.

Before I lost the weight, I always used to fade to the back. But now, I want to be out in the front. Now, I’m more bubbly and outgoing.
Verified Patient
Pauline C.

You feel so much better when you hop out of the shower and you look at yourself, and you’re like, ‘oh, what do you know? My belly went down.’
Verified Patient
Kris R.

The results are astonishing. I’ve lost so much weight and I didn't think it was possible at my age. I’m very happy that I found this program.
Verified Patient
Ismael F.

Every morning, I look forward to getting on the scale. I’ve been on it for five to six weeks and I’ve lost 17 pounds so far.
Verified Patient
Jamie R.

I’ve been on the medication for about six weeks and I’ve lost 20 pounds!
Verified Patient
Blake T.

My clothes are fitting the way they should, and I’m losing the extra fluff around the muscle. I just feel way more comfortable in myself.
Verified Patient
Tara O.

Reviews shown are from verified LifeMD patients across various services. Photos are for illustrative purposes only.
Weight Loss Money Back Guarantee
Lose 10% of your weight or your money back+
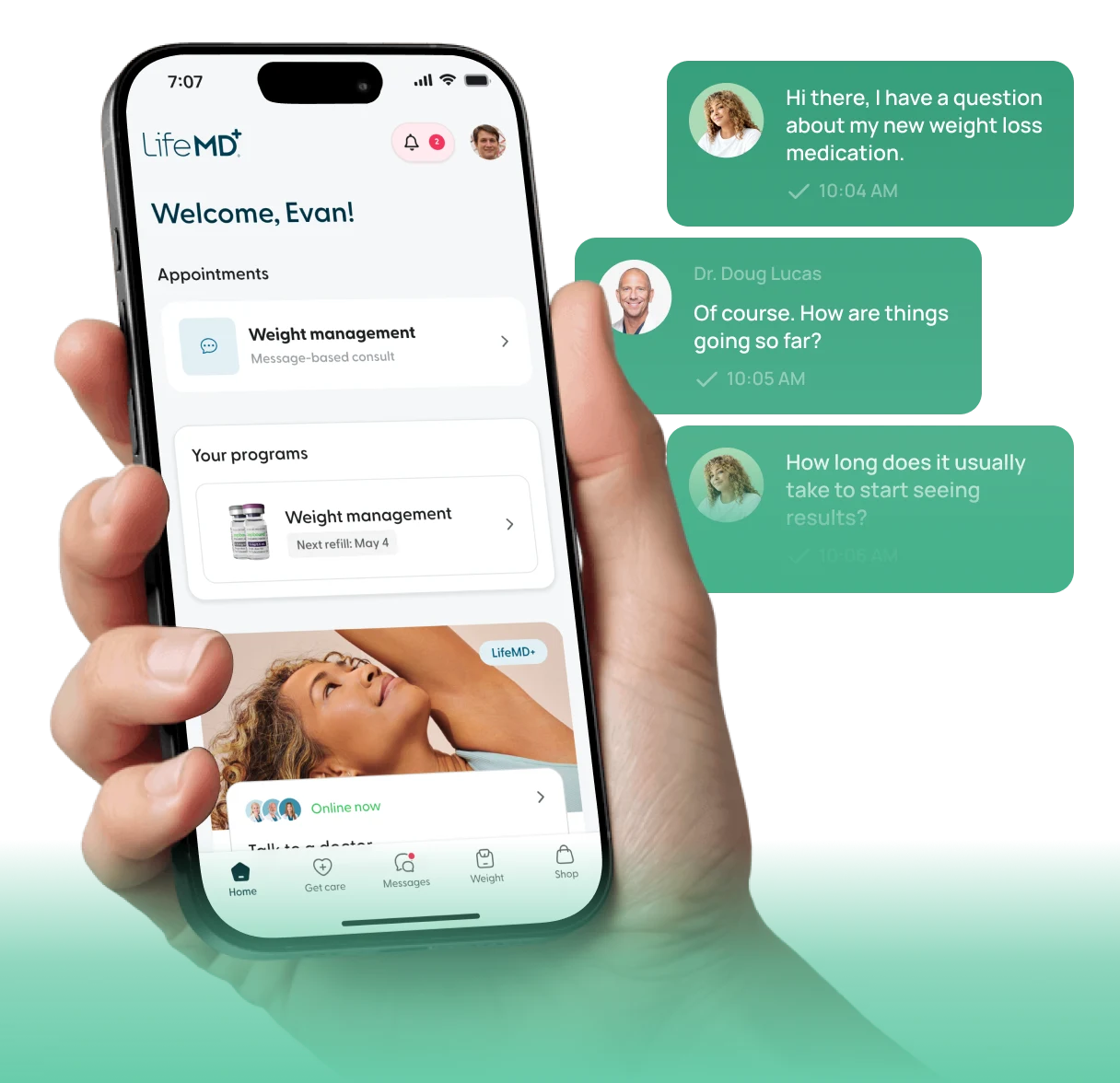
Table of Contents
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg and Wegovy® (semaglutide) tablets 25 mg are prescription medicines used with a reduced calorie diet and increased physical activity to:
reduce the risk of major cardiovascular events such as death, heart attack, or stroke in adults with known heart disease and with either obesity or overweight.
help adults with obesity, or some adults with excess weight (overweight) who also have weight-related medical problems to lose weight and keep the weight off.
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg is used with a reduced-calorie diet and increased physical activity to help children 12 years and older with obesity to lose weight and keep the weight off.
Wegovy® contains semaglutide and should not be used with other semaglutide-containing products or other GLP-1 receptor agonist medicines.
It is not known if Wegovy® injection is safe and effective:
to reduce the risk of major cardiovascular events (death, heart attack, or stroke) in people under 18 years
to help children under 12 years of age lose weight and keep the weight off
It is not known if Wegovy® tablets are safe and effective for use in people under 18 years of age.
Important Safety Information
What is the most important information I should know about Wegovy®?
Wegovy® may cause serious side effects, including:
Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rodents, Wegovy® and other medicines that work like Wegovy® caused thyroid tumors, including thyroid cancer. It is not known if Wegovy® will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people
Do not use Wegovy® if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
Do not use Wegovy® if:
you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
you have had a serious allergic reaction to semaglutide or any of the ingredients in Wegovy® injection or Wegovy® tablets. See symptoms of serious allergic reaction in "What are the possible side effects of Wegovy®?"
Before using Wegovy®, tell your healthcare provider if you have any other medical conditions, including if you:
have or have had problems with your pancreas or kidneys
have type 2 diabetes and a history of diabetic retinopathy
have or have had depression, suicidal thoughts, or mental health issues
are scheduled to have surgery or other procedures that use anesthesia or deep sleepiness (deep sedation)
are pregnant or plan to become pregnant. Wegovy® may harm your unborn baby. You should stop using Wegovy® 2 months before you plan to become pregnant
are breastfeeding or plan to breastfeed. Breastfeeding is not recommended during treatment with Wegovy® tablets. It is not known if Wegovy® when received through an injection passes into your breast milk
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Wegovy® may affect the way some medicines work and some medicines may affect the way Wegovy® works. Tell your healthcare provider if you are taking other medicines to treat diabetes, including sulfonylureas or insulin. Wegovy® slows stomach emptying and can affect medicines that need to pass through the stomach quickly.
What are the possible side effects of Wegovy®?
Wegovy® may cause serious side effects, including:
inflammation of your pancreas (pancreatitis). Stop using Wegovy® and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without nausea or vomiting. Sometimes you may feel the pain from your abdomen to your back
gallbladder problems. Wegovy® may cause gallbladder problems, including gallstones. Some gallstones may need surgery. Call your healthcare provider if you have symptoms, such as pain in your upper stomach (abdomen), fever, yellowing of the skin or eyes (jaundice), or clay-colored stools
increased risk of low blood sugar (hypoglycemia), especially those who also take medicines for diabetes such as insulin or sulfonylureas. This can be a serious side effect. Talk to your healthcare provider about how to recognize and treat low blood sugar and check your blood sugar before you start and while you take Wegovy®. Signs and symptoms of low blood sugar may include dizziness or light-headedness, blurred vision, anxiety, irritability or mood changes, sweating, slurred speech, hunger, confusion or drowsiness, shakiness, weakness, headache, fast heartbeat, or feeling jittery
dehydration leading to kidney problems. Diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems. It is important for you to drink fluids to help reduce your chance of dehydration. Tell your healthcare provider right away if you have nausea, vomiting, or diarrhea that does not go away
severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Wegovy®. Tell your healthcare provider if you have stomach problems that are severe or will not go away
serious allergic reactions. Stop using Wegovy® and get medical help right away, if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue, or throat; problems breathing or swallowing; severe rash or itching; fainting or feeling dizzy; or very rapid heartbeat
change in vision in people with type 2 diabetes. Tell your healthcare provider if you have changes in vision during treatment with Wegovy®
increased heart rate. Wegovy® can increase your heart rate while you are at rest. Tell your healthcare provider if you feel your heart racing or pounding in your chest and it lasts for several minutes
depression or thoughts of suicide. You should pay attention to any mental changes, especially sudden changes in your mood, behaviors, thoughts, or feelings. Call your healthcare provider right away if you have any mental changes that are new, worse, or worry you
food or liquid getting into the lungs during surgery or other procedures that use anesthesia or deep sleepiness (deep sedation). Wegovy® may increase the chance of food getting into your lungs during surgery or other procedures. Tell all your healthcare providers that you are taking Wegovy® before you are scheduled to have surgery or other procedures
The most common side effects of Wegovy® may include: nausea, diarrhea, vomiting, constipation, stomach (abdomen) pain, headache, tiredness (fatigue), upset stomach, dizziness, feeling bloated, belching, low blood sugar in people with type 2 diabetes, gas, stomach flu, heartburn, and runny nose or sore throat.
Please see Prescribing Information and Medication Guide for Wegovy®.
Wegovy® is a prescription medication.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
This site is intended for US patients only.
RYBELSUS® (semaglutide) tablets 7 mg or 14 mg is a prescription medicine used along with diet and exercise to improve blood sugar (glucose) in adults with type 2 diabetes.
It is not known if RYBELSUS® can be used in people who have had pancreatitis
RYBELSUS® is not for use in people with type 1 diabetes
It is not known if RYBELSUS® is safe and effective for use in children under 18 years of age
Important Safety Information
What is the most important information I should know about RYBELSUS®?
RYBELSUS® may cause serious side effects, including:
Possible thyr oid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyr oid cancer. In studies with rodents, RYBELSUS® and medicines that work like RYBELSUS® caused thyr oid tumors, including thyr oid cancer. It is not known if RYBELSUS® will cause thyr oid tumors or a type of thyr oid cancer called medullary thyr oid carcinoma (MTC) in people
Do not use RYBELSUS® if:
you or any of your family have ever had MTC, or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
you have had a serious allergic reaction to semaglutide or any of the ingredients in RYBELSUS®. See symptoms of serious allergic reaction in "What are the possible side effects of RYBELSUS®?"
Before using RYBELSUS®, tell your healthcare provider if you have any other medical conditions, including if you:
have or have had problems with your pancreas or kidneys
have a history of vision problems related to your diabetes
are pregnant or plan to become pregnant. It is not known if RYBELSUS® will harm your unborn baby. You should stop using RYBELSUS® 2 months before you plan to become pregnant. Talk to your healthcare provider about the best way to control your blood sugar if you plan to become pregnant or while you are pregnant
are breastfeeding or plan to breastfeed. Breastfeeding is not recommended during treatment with RYBELSUS®
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. RYBELSUS® may affect the way some medicines work and some medicines may affect the way RYBELSUS® works.
How should I take RYBELSUS®?
Take RYBELSUS® exactly as your healthcare provider tells you to
Take RYBELSUS® by mouth on an empty stomach when you first wake up with a sip of plain water (no more than 4 ounces)
Do not split, crush, or chew. Swallow RYBELSUS® whole
After 30 minutes, you can eat, drink, or take other oral medicines
If you miss a dose of RYBELSUS®, skip the missed dose and go back to your regular schedule
What are the possible side effects of RYBELSUS®?
RYBELSUS® may cause serious side effects, including:
inflammation of your pancreas (pancreatitis). Stop using RYBELSUS® and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back
changes in vision. Tell your healthcare provider if you have changes in vision during treatment with RYBELSUS®
low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use RYBELSUS® with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. Signs and symptoms of low blood sugar may include: dizziness or lightheadedness, blurred vision, anxiety, irritability or mood changes, sweating, slurred speech, hunger, confusion or drowsiness, shakiness, weakness, headache, fast heartbeat, and feeling jittery
kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems to get worse. It is important for you to drink fluids to help reduce your chance of dehydration
serious allergic reactions. Stop using RYBELSUS® and get medical help right away, if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue, or throat; problems breathing or swallowing; severe rash or itching; fainting or feeling dizzy; or very rapid heartbeat
gallbladder problems. Gallbladder problems have happened in some people who take RYBELSUS®. Tell your healthcare provider right away if you get symptoms of gallbladder problems, which may include: pain in your upper stomach (abdomen), yellowing of skin or eyes (jaundice), fever, and clay-colored stools
The most common side effects of RYBELSUS® may include nausea, stomach (abdominal) pain, diarrhea, decreased appetite, vomiting, and constipation. Nausea, vomiting, and diarrhea are most common when you first start RYBELSUS®.
Please see Prescribing Information and Medication Guide for RYBELSUS®.
RYBELSUS® is a prescription medication.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Saxenda® (liraglutide) injection 3 mg is an injectable prescription medicine used for adults with excess weight (BMI ≥27) who also have weight-related medical problems or obesity (BMI ≥30), and children aged 12-17 years with a body weight above 132 pounds (60 kg) and obesity to help them lose weight and keep the weight off. Saxenda® should be used with a reduced calorie diet and increased physical activity.
Saxenda® and Victoza® have the same active ingredient, liraglutide, and should not be used together or with other GLP-1 receptor agonist medicines
It is not known if Saxenda® is safe and effective when taken with other prescription, over-the-counter medicines, or herbal weight-loss products
It is not known if Saxenda® is safe and effective in children under 12 years of age
It is not known if Saxenda® is safe and effective in children aged 12 to 17 years with type 2 diabetes
Do not share your Saxenda® pen with others even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
What is the most important information I should know about Saxenda®?
Serious side effects may happen in people who take Saxenda®, including:
Possible thyr oid tumors, including cancer. Tell your health care professional if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyr oid cancer. In studies with rats and mice, Saxenda® and medicines that work like Saxenda® caused thyr oid tumors, including thyr oid cancer. It is not known if Saxenda® will cause thyr oid tumors or a type of thyr oid cancer called medullary thyr oid carcinoma (MTC) in people.
Do not use Saxenda® if you or any of your family have ever had MTC, or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Who should not use Saxenda®?
Do not use Saxenda® if:
you or any of your family have ever had MTC or if you have MEN 2
you have had a serious allergic reaction to liraglutide or any of the ingredients in Saxenda®. See symptoms of serious allergic reactions in "What are the possible side effects of Saxenda®?"
you are pregnant or plan to become pregnant. Saxenda® may harm your unborn baby
Before taking Saxenda®, tell your health care provider about all of your medical conditions, including if you:
are taking certain medicines called GLP-1 receptor agonists
have severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food
have or have had problems with your pancreas, kidneys or liver
have or have had depression or suicidal thoughts, or mental health issues
are breastfeeding or plan to breastfeed. It is not known if Saxenda® passes into your breast milk. You and your health care provider should decide if you will use Saxenda® or breastfeed
Tell your health care provider about all the medicines you take, including prescription, over-the-counter medicines, vitamins, and herbal supplements. Saxenda® slows stomach emptying and can affect medicines that need to pass through the stomach quickly. Saxenda® may affect the way some medicines work and some other medicines may affect the way Saxenda® works. Tell your health care provider if you take diabetes medicines, especially insulin and sulfonylurea medicines.
How should I use Saxenda®?
Read the Instructions for Use that comes with Saxenda®
Inject your dose of Saxenda® under the skin (subcutaneously) in your stomach area (abdomen), upper leg (thigh), or upper arm, as instructed by your health care provider. Do not inject into a vein or muscle
Change (rotate) your injection site within the area you choose with each injection to reduce your risk of getting lumps under the skin (cutaneous amyloidosis). Do not use the same site for each injection
What are the possible side effects of Saxenda®?
Saxenda® may cause serious side effects, including:
inflammation of the pancreas (pancreatitis). Stop using Saxenda® and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your stomach area (abdomen) to your back
gallbladder problems. Saxenda® may cause gallbladder problems, including gallstones. Some gallbladder problems need surgery. Call your health care provider if you have any of the following symptoms: pain in your upper stomach (abdomen), fever, yellowing of your skin or eyes (jaundice), or clay-colored stools
increased risk of low blood sugar (hypoglycemia) in adults with type 2 diabetes who also take medicines to treat type 2 diabetes such as sulfonylureas or insulin
risk of low blood sugar (hypoglycemia) in children who are 12 years of age and older without type 2 diabetes
Signs and symptoms of low blood sugar may include: shakiness, sweating, headache, drowsiness, weakness, dizziness, confusion, irritability, hunger, fast heartbeat, and feeling jittery. You should check your blood sugar before you start taking Saxenda® and while you take Saxenda®
increased heart rate. Saxenda® can increase your heart rate while you are at rest. Your health care provider should check your heart rate while you take Saxenda®. Tell your health care professional if you feel your heart racing or pounding in your chest and it lasts for several minutes
kidney problems (kidney failure). Saxenda® may cause nausea, vomiting, or diarrhea leading to loss of fluids (dehydration). Dehydration may cause kidney failure, which can lead to the need for dialysis. This can happen in people who have never had kidney problems before. Drinking plenty of fluids may reduce your chance of dehydration. Call your health care provider right away if you have nausea, vomiting, or diarrhea that does not go away, or if you cannot drink liquids by mouth
serious allergic reactions. Stop using Saxenda® and get medical help right away if you have any symptoms of a serious allergic reaction including swelling of your face, lips, tongue, or throat, fainting or feeling dizzy, very rapid heartbeat, problems breathing or swallowing, or severe rash or itching
depression or thoughts of suicide. You should pay attention to any mental changes, especially sudden changes, in your mood, behaviors, thoughts, or feelings. Call your health care provider right away if you have any mental changes that are new, worse, or worry you
The most common side effects of Saxenda® in adults include nausea, diarrhea, constipation, vomiting, injection site reaction, low blood sugar (hypoglycemia), headache, tiredness (fatigue), dizziness, stomach pain, and change in enzyme (lipase) levels in your blood. Additional common side effects in children are fever and gastroenteritis.
Please click here for Prescribing Information and Medication Guide for Saxenda®.
Saxenda® is a prescription medication.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Ozempic® may cause serious side effects, including:
Possible thyr oid tumors, including cancer. Tell your health care provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyr oid cancer. In studies with rodents, Ozempic® and medicines that work like Ozempic® caused thyr oid tumors, including thyr oid cancer. It is not known if Ozempic® will cause thyr oid tumors or a type of thyr oid cancer called medullary thyr oid carcinoma (MTC) in people.
Do not use Ozempic® if you or any of your family have ever had MTC, or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Do not use Ozempic® if:
you or any of your family have ever had MTC or if you have MEN 2.
you are allergic to semaglutide or any of the ingredients in Ozempic®. See symptoms of serious allergic reaction in "What are the possible side effects of Ozempic®?".
Before using Ozempic®, tell your health care provider if you have any other medical conditions, including if you:
have or have had problems with your pancreas or kidneys.
have a history of diabetic retinopathy.
are pregnant or breastfeeding or plan to become pregnant or breastfeed. It is not known if Ozempic® will harm your unborn baby or passes into your breast milk. You should stop using Ozempic® 2 months before you plan to become pregnant.
Tell your health care provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, herbal supplements, and other medicines to treat diabetes, including insulin or sulfonylureas.
What are the possible side effects of Ozempic®?
Ozempic® may cause serious side effects, including:
inflammation of your pancreas (pancreatitis). Stop using Ozempic® and call your health care provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
changes in vision. Tell your health care provider if you have changes in vision during treatment with Ozempic®.
low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Ozempic® with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. Signs and symptoms of low blood sugar may include: dizziness or Iightheadedness, blurred vision, anxiety, irritability or mood changes, sweating, slurred speech, hunger, confusion or drowsiness, shakiness, weakness, headache, fast heartbeat, and feeling jittery.
kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems to get worse. It is important for you to drink fluids to help reduce your chance of dehydration.
serious allergic reactions. Stop using Ozempic® and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue, or throat; problems breathing or swallowing; severe rash or itching; fainting or feeling dizzy; or very rapid heartbeat.
gallbladder problems. Gallbladder problems have happened in some people who take Ozempic®. Tell your healthcare provider right away if you get symptoms which may include: pain in your upper stomach (abdomen), fever, yellowing of the skin or eyes (jaundice), or clay-colored stools.
The most common side effects of Ozempic® may include nausea, vomiting, diarrhea, stomach (abdominal) pain, and constipation.
Please click here for Prescribing Information and Medication Guide.
Ozempic® is a prescription medication.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Victoza® may cause serious side effects, including:
Possible thyr oid tumors, including cancer. Tell your health care provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyr oid cancer. In studies with rats and mice, Victoza® and medicines that work like Victoza® caused thyr oid tumors, including thyr oid cancer. It is not known if Victoza® will cause thyr oid tumors or a type of thyr oid cancer called medullary thyr oid carcinoma (MTC) in people.
Who should not use Victoza®?
Do not use Victoza® if:
you or any of your family have ever had MTC or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
you are allergic to liraglutide or any of the ingredients in Victoza®. See symptoms of serious allergic reaction in “What are the possible side effects of Victoza®?”.
What is Victoza®?
Victoza® (liraglutide) injection 1.2 mg or 1.8 mg is an injectable prescription medicine used:
along with diet and exercise to lower blood sugar (glucose) in adults and children who are 10 years of age and older with type 2 diabetes mellitus.
to reduce the risk of major cardiovascular events such as heart attack, stroke, or death in adults with type 2 diabetes mellitus with known heart disease.
Victoza® is not for use in people with type 1 diabetes. It should not be used with other medicines that contain liraglutide. It is not known if Victoza® is safe and effective to lower blood sugar in children under 10 years of age.
Important Safety Information
What should I tell my healthcare provider before using Victoza®?
Before using Victoza®, tell your health care provider if you:
have or have had problems with your pancreas, kidneys, or liver.
have any other medical conditions or severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food.
are pregnant or breastfeeding or plan to become pregnant or breastfeed.
Tell your health care provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, herbal supplements, and other medicines to treat diabetes, including insulin or sulfonylureas.
How should I use Victoza®?
Inject under the skin of your stomach (abdomen), thigh, or upper arm. Do not inject Victoza® into a muscle or vein.
Change (rotate) your injection site within the area you choose with each injection to reduce your risk of getting lumps under the skin (cutaneous amyloidosis). Do not use the same site for each injection.
Do not mix insulin and Victoza® together in the same injection.
You may give an injection of Victoza® and insulin in the same body area (such as your stomach area), but not right next to each other.
Do not share your Victoza® pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
What are the possible side effects of Victoza®?
Victoza® may cause serious side effects, including:
inflammation of your pancreas (pancreatitis). Stop using Victoza® and call your health care provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Victoza® with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. In children who are 10 years of age and older, the risk for low blood sugar may be higher with Victoza® regardless of use with another medicine that can also lower blood sugar. Signs and symptoms of low blood sugar may include: dizziness or lightheadedness, blurred vision, anxiety, irritability or mood changes, sweating, slurred speech, hunger, confusion or drowsiness, shakiness, weakness, headache, fast heartbeat, and feeling jittery.
kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems to get worse.
serious allergic reactions. Stop using Victoza® and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue or throat, problems breathing or swallowing, severe rash or itching, fainting or feeling dizzy, or a very rapid heartbeat.
gallbladder problems. Gallbladder problems have happened in some people who take Victoza®. Tell your healthcare provider right away if you get symptoms of gallbladder problems, which may include pain in the upper stomach (abdomen), fever, yellowing of skin or eyes (jaundice), or clay-colored stools.
The most common side effects of Victoza® may include nausea, diarrhea, vomiting, decreased appetite, indigestion, and constipation.
Please click here for Prescribing Information and Medication Guide.
Victoza® is a prescription medication.
Trulicity® is for adults and children 10 years of age and older with type 2 diabetes used along with diet and exercise to improve blood sugar (glucose). Trulicity® is also used in adults with type 2 diabetes to reduce the risk of major cardiovascular events (problems having to do with the heart and blood vessels) such as death, heart attack, or stroke in people who have heart disease or multiple cardiovascular risk factors.
It is not known if TRULICITY® can be used in people who have had inflammation of the pancreas (pancreatitis). TRULICITY® is not for use in people with type 1 diabetes and is not recommended for use in people with severe stomach or intestinal problems. It is not known if TRULICITY® is safe and effective in children under 10 years of age.
Trulicity® is given through an injection (needle). You take it once a week by injecting it under the skin of your stomach, thigh, or upper arm.
Warnings: Trulicity® may cause tumors in the thyr oid , including thyr oid cancer. Watch for possible symptoms, such as a lump or swelling in the neck, trouble swallowing, hoarseness, or shortness of breath. If you have any of these symptoms, tell your healthcare provider.
Do not use Trulicity® if you or any of your family have ever had a type of thyr oid cancer called medullary thyr oid carcinoma (MTC).
Do not use Trulicity® if you have Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Do not use Trulicity® if you are allergic to dulaglutide or other ingredients in Trulicity®.
Ask your healthcare provider how to recognize possible serious side effects and what to do :
Inflamed pancreas (pancreatitis). Stop using Trulicity® and call your healthcare provider right away if you have severe pain in your stomach area (abdomen), with or without vomiting, that will not go away. You may feel the pain from your abdomen to your back.
Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use TRULICITY® with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin.
Signs and symptoms of low blood sugar may include dizziness or light-headedness, confusion or drowsiness, headache, blurred vision, slurred speech, fast heartbeat, sweating, hunger, shakiness, feeling jittery, weakness, anxiety, irritability, or mood changes.
Serious allergic reactions. Stop using Trulicity® and get medical help right away if you have any symptoms of a serious allergic reaction which may include swelling of your face, lips, tongue or throat, problems breathing or swallowing, severe rash or itching, fainting, or feeling dizzy, or very rapid heartbeat.
Acute kidney injury. In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration). This may cause kidney problems to get worse.
Severe stomach problems. Trulicity® may cause stomach problems, which could be severe.
Changes in vision. Tell your healthcare provider if you have changes in your eyesight (vision) during treatment with Trulicity®.
Gallbladder problems. Gallbladder problems have happened in some people who take Trulicity®. Tell your healthcare provider right away if you get symptoms of gallbladder problems, which may include pain in your upper stomach (abdomen), fever, yellowing of skin or eyes (jaundice), clay-colored stools.
Common side effects
The most common side effects of Trulicity® include nausea, diarrhea, vomiting, abdominal pain and decreased appetite, indigestion, and fatigue.
These are not all the possible side effects of Trulicity®.
Tell your healthcare provider if you have any side effects. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before using
Your healthcare provider should show you how to use Trulicity® before you use it for the first time.
Before you use Trulicity®, talk to your healthcare provider about low blood sugar and how to manage it.
Review these questions with your healthcare provider:
Do you have other medical conditions, including problems with your pancreas, kidneys, liver, or stomach, or have a history of diabetic retinopathy (vision problems related to diabetes)?
Do you take other diabetes medicines, such as insulin or sulfonylureas?
Are you pregnant or plan to become pregnant or breastfeeding or plan to breastfeed?
Do you take any other prescription medicines or over-the-counter drugs, vitamins, or herbal supplements?
How to take
Read the Instructions for Use that come with Trulicity®.
Use Trulicity® exactly as your healthcare provider says.
Do not share your Trulicity® pen, syringe, or needles with another person.
Do not give Trulicity® to other people.
If you take too much Trulicity®, call your healthcare provider or seek medical advice promptly.
Trulicity® is a prescription medicine. For more information, call 1-844-TRU-INFO (1-844-878-4636) or go to www.TRULICITY®.com.
This summary provides basic information about Trulicity® but does not include all information known about this medicine. Read the information that comes with your prescription each time your prescription is filled. This information does not take the place of talking with your healthcare provider. Be sure to talk to your healthcare provider about Trulicity® and how to take it. Your healthcare provider is the best person to help you decide if Trulicity® is right for you.
Warnings: Zepbound may cause tumors in the thyr oid , including thyr oid cancer. Watch for possible symptoms, such as a lump or swelling in the neck, hoarseness, trouble swallowing, or shortness of breath. If you have any of these symptoms, tell your healthcare provider.
Do not use Zepbound if you or any of your family have ever had a type of thyr oid cancer called medullary thyr oid carcinoma (MTC).
Do not use Zepbound Do not use Zepbound if you have Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Do not use Zepbound if you have had a serious allergic reaction to tirzepatide or any of the ingredients in Zepbound.
Zepbound may cause serious side effects, including:
Severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Zepbound. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
Kidney problems (kidney failure). Diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems. It is important for you to drink fluids to help reduce your chance of dehydration.
Gallbladder problems. Gallbladder problems have happened in some people who use Zepbound. Tell your healthcare provider right away if you get symptoms of gallbladder problems, which may include pain in your upper stomach (abdomen), fever, yellowing of skin or eyes (jaundice), or clay-colored stools.
Inflammation of the pancreas (pancreatitis). Stop using Zepbound and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
Serious allergic reactions. Stop using Zepbound and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue or throat, problems breathing or swallowing, severe rash or itching, fainting or feeling dizzy, or very rapid heartbeat.
Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Zepbound with medicines that can cause low blood sugar, such as a sulfonylurea or insulin.
Signs and symptoms of low blood sugar may include dizziness or light-headedness, sweating, confusion or drowsiness, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability, mood changes, hunger, weakness or feeling jittery.
Changes in vision in patients with type 2 diabetes. Tell your healthcare provider if you have changes in vision during treatment with Zepbound.
Depression or thoughts of suicide. You should pay attention to changes in your mood, behaviors, feelings or thoughts. Call your healthcare provider right away if you have any mental changes that are new, worse, or worry you.
Common side effects
The most common side effects of Zepbound include nausea, diarrhea, vomiting, constipation, stomach (abdominal) pain, indigestion, injection site reactions, feeling tired, allergic reactions, belching, hair loss, and heartburn. These are not all the possible side effects of Zepbound. Talk to your healthcare provider about any side effect that bothers you or doesn’t go away.
Tell your healthcare provider if you have any side effects. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before using Zepbound
Your healthcare provider should show you how to use Zepbound before you use it for the first time.
Tell your healthcare provider if you are taking medicines to treat diabetes including insulin or sulfonylureas which could increase your risk of low blood sugar. Talk to your healthcare provider about low blood sugar levels and how to manage them.
If you take birth control pills by mouth, talk to your healthcare provider before you use Zepbound. Birth control pills may not work as well while using Zepbound. Your healthcare provider may recommend another type of birth control for 4 weeks after you start Zepbound and for 4 weeks after each increase in your dose of Zepbound.
Review these questions with your healthcare provider:
Do you have other medical conditions, including problems with your pancreas or kidneys, or severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems digesting food?
Do you take other diabetes medicines, such as insulin or sulfonylureas?
Do you have a history of diabetic retinopathy?
Do you take any other prescription medicines or over-the-counter drugs, vitamins, or herbal supplements?
Are you pregnant, plan to become pregnant, breastfeeding, or plan to breastfeed? Zepbound may harm your unborn baby. Tell your healthcare provider if you become pregnant while using Zepbound. It is not known if Zepbound passes into your breast milk. You should talk with your healthcare provider about the best way to feed your baby while using Zepbound.
How to take
Read the Instructions for Use that come with Zepbound.
Use Zepbound exactly as your healthcare provider says.
Zepbound is injected under the skin (subcutaneously) of your stomach (abdomen), thigh, or upper arm.
Use Zepbound 1 time each week, at any time of the day.
Change (rotate) your injection site with each weekly injection. Do not use the same site for each injection.
If you take too much Zepbound, call your healthcare provider, seek medical advice promptly, or contact a Poison Center expert right away at 1-800-222-1222.
Learn more
Zepbound is a prescription medicine. For more information, call 1-800-LillyRx (1-800-545-5979) or go to www.zepbound.lilly.com.
Warnings: Mounjaro® may cause tumors in the thyr oid , including thyr oid cancer. Watch for possible symptoms, such as a lump or swelling in the neck, hoarseness, trouble swallowing, or shortness of breath. If you have any of these symptoms, tell your healthcare provider.
Do not use Mounjaro if you or any of your family have ever had a type of thyr oid cancer called medullary thyr oid carcinoma (MTC).
Do not use Mounjaro if you have Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Do not use Mounjaro if you are allergic to it or any of the ingredients in Mounjaro.
Mounjaro may cause serious side effects, including:
Inflammation of the pancreas (pancreatitis). Stop using Mounjaro and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Mounjaro with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. Signs and symptoms of low blood sugar may include dizziness or light-headedness, sweating, confusion or drowsiness, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability, or mood changes, hunger, weakness and feeling jittery.
Serious allergic reactions. Stop using Mounjaro and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue or throat, problems breathing or swallowing, severe rash or itching, fainting or feeling dizzy, and very rapid heartbeat.
Kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems to get worse. It is important for you to drink fluids to help reduce your chance of dehydration.
Severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Mounjaro. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
Changes in vision. Tell your healthcare provider if you have changes in vision during treatment with Mounjaro.
Gallbladder problems. Gallbladder problems have happened in some people who use Mounjaro. Tell your healthcare provider right away if you get symptoms of gallbladder problems, which may include pain in your upper stomach (abdomen), fever, yellowing of skin or eyes (jaundice), and clay-colored stools.
Common side effects
The most common side effects of Mounjaro include nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion, and stomach (abdominal) pain. These are not all the possible side effects of Mounjaro. Talk to your healthcare provider about any side effect that bothers you or doesn’t go away.
Tell your healthcare provider if you have any side effects. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before using Mounjaro
Your healthcare provider should show you how to use Mounjaro before you use it for the first time.
Talk to your healthcare provider about low blood sugar and how to manage it.
If you take birth control pills by mouth, talk to your healthcare provider before you use Mounjaro. Birth control pills may not work as well while using Mounjaro. Your healthcare provider may recommend another type of birth control for 4 weeks after you start Mounjaro and for 4 weeks after each increase in your dose of Mounjaro.
Review these questions with your healthcare provider:
Do you have other medical conditions, including problems with your pancreas or kidneys, or severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems digesting food?
Do you take other diabetes medicines, such as insulin or sulfonylureas?
Do you have a history of diabetic retinopathy?
Are you pregnant, plan to become pregnant, breastfeeding, or plan to breastfeed? It is not known if Mounjaro will harm your unborn baby or pass into your breast milk.
Do you take any other prescription medicines or over-the-counter drugs, vitamins, or herbal supplements?
How to take
Read the Instructions for Use that come with Mounjaro.
Use Mounjaro exactly as your healthcare provider says.
Mounjaro is injected under the skin (subcutaneously) of your stomach (abdomen), thigh, or upper arm.
Use Mounjaro 1 time each week, at any time of the day.
Do not mix insulin and Mounjaro together in the same injection.
You may give an injection of Mounjaro and insulin in the same body area (such as your stomach area), but not right next to each other.
Change (rotate) your injection site with each weekly injection. Do not use the same site for each injection.
If you take too much Mounjaro, call your healthcare provider or seek medical advice promptly.
Learn more
Mounjaro is a prescription medicine. For more information, call 1-833-807-MJRO (833-807-6576) or go to www.mounjaro.com.
Important Safety Information
What is the most important information I should know about Semaglutide?
Semaglutide may cause serious side effects, including:
Possible thyr oid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyr oid cancer. In studies with rodents, Semaglutide and medicines that work like Semaglutide caused thyr oid tumors, including thyr oid cancer. It is not known if Semaglutide will cause thyr oid tumors or a type of thyr oid cancer called medullary thyr oid carcinoma (MTC) in people
Do not use Semaglutide if you or any of your family have ever had a type of thyr oid cancer called medullary thyr oid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
Do not use Semaglutide if:
you or any of your family have ever had a type of thyr oid cancer called medullary thyr oid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
you have had a serious allergic reaction to semaglutide or any of the ingredients in Semaglutide
Before using Semaglutide, tell your healthcare provider if you have any other medical conditions, including if you:
have or have had problems with your pancreas or kidneys
have type 2 diabetes and a history of diabetic retinopathy
have or have had depression, suicidal thoughts, or mental health issues
are pregnant or plan to become pregnant. Semaglutide may harm your unborn baby. You should stop using Semaglutide 2 months before you plan to become pregnant
are breastfeeding or plan to breastfeed. It is not known if Semaglutide passes into your breast milk
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Semaglutide may affect the way some medicines work and some medicines may affect the way Semaglutide works. Tell your healthcare provider if you are taking other medicines to treat diabetes, including sulfonylureas or insulin. Semaglutide slows stomach emptying and can affect medicines that need to pass through the stomach quickly.
What are the possible side effects of Semaglutide?
Semaglutide may cause serious side effects, including:
inflammation of your pancreas (pancreatitis). Stop using Semaglutide and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back
gallbladder problems. Semaglutide may cause gallbladder problems, including gallstones. Some gallstones may need surgery. Call your healthcare provider if you have symptoms, such as pain in your upper stomach (abdomen), fever, yellowing of the skin or eyes (jaundice), or clay-colored stools
increased risk of low blood sugar (hypoglycemia) in patients with type 2 diabetes, especially those who also take medicines for type 2 diabetes such as sulfonylureas or insulin. This can be both a serious and common side effect. Talk to your healthcare provider about how to recognize and treat low blood sugar and check your blood sugar before you start and while you take Semaglutide. Signs and symptoms of low blood sugar may include dizziness or light-headedness, blurred vision, anxiety, irritability or mood changes, sweating, slurred speech, hunger, confusion or drowsiness, shakiness, weakness, headache, fast heartbeat, or feeling jittery
kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems to get worse. It is important for you to drink fluids to help reduce your chance of dehydration
serious allergic reactions. Stop using Semaglutide and get medical help right away, if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue, or throat; problems breathing or swallowing; severe rash or itching; fainting or feeling dizzy; or very rapid heartbeat
change in vision in people with type 2 diabetes. Tell your healthcare provider if you have changes in vision during treatment with Semaglutide
increased heart rate. Semaglutide can increase your heart rate while you are at rest. Tell your healthcare provider if you feel your heart racing or pounding in your chest and it lasts for several minutes
depression or thoughts of suicide. You should pay attention to any mental changes, especially sudden changes in your mood, behaviors, thoughts, or feelings. Call your healthcare provider right away if you have any mental changes that are new, worse, or worry you
The most common side effects of Semaglutide may include: nausea, diarrhea, vomiting, constipation, stomach (abdomen) pain, headache, tiredness (fatigue), upset stomach, dizziness, feeling bloated, belching, gas, stomach flu, heartburn, and runny nose or sore throat.
Semaglutide is a prescription medication.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
What is the most important information I should know about Tirzepatide? Tirzepatide may cause serious side effects, including:
Possible thyr oid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyr oid cancer. In studies with rats, Tirzepatide and medicines that work like Tirzepatide caused thyr oid tumors, including thyr oid cancer. It is not known if Tirzepatide will cause thyr oid tumors, or a type of thyr oid cancer called medullary thyr oid carcinoma (MTC) in people. Do not use Tirzepatide if you or any of your family have ever had a type of thyr oid cancer called medullary thyr oid carcinoma (MTC), or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Tirzepatide may cause serious side effects, including:
Inflammation of the pancreas (pancreatitis). Stop using Tirzepatide and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Tirzepatide with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. Signs and symptoms of low blood sugar may include dizziness or light-headedness, sweating, confusion or drowsiness, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability, or mood changes, hunger, weakness and feeling jittery.
Serious allergic reactions. Stop using Tirzepatide and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue or throat, problems breathing or swallowing, severe rash or itching, fainting or feeling dizzy, and very rapid heartbeat.
Kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems to get worse. It is important for you to drink fluids to help reduce your chance of dehydration.
Severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Tirzepatide. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
Changes in vision. Tell your healthcare provider if you have changes in vision during treatment with Tirzepatide.
Gallbladder problems. Gallbladder problems have happened in some people who use Tirzepatide. Tell your healthcare provider right away if you get symptoms of gallbladder problems, which may include pain in your upper stomach (abdomen), fever, yellowing of skin or eyes (jaundice), and clay-colored stools.
Common side effects
The most common side effects of Tirzepatide include nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion, and stomach (abdominal) pain. These are not all the possible side effects of Tirzepatide. Talk to your healthcare provider about any side effect that bothers you or doesn’t go away.
Tell your healthcare provider if you have any side effects. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.
This summary provides basic information about Tirzepatide but does not include all information known about this medicine. Read the information that comes with your prescription each time your prescription is filled. This information does not take the place of talking with your healthcare provider. Be sure to talk to your healthcare provider about Tirzepatide and how to take it. Your healthcare provider is the best person to help you decide if Tirzepatide is right for you.
Important Safety Information
Orlistat is contraindicated in patients:
who are pregnant. Weight loss offers no potential benefit to a pregnant woman and may result in fetal harm. A minimum weight gain, and no weight loss, is currently recommended for all pregnant women, including those who are already overweight or obese, due to the obligatory weight gain that occurs in maternal tissues during pregnancy
with chronic malabsorption syndrome
with cholestasis
with known hypersensitivity to Orlistat or to any component of this product
Warnings and Precautions:
Orlistat may interact with concomitant drugs including cyclosporine, levothyroxine, warfarin, amiodarone, antiepileptic drugs, and antiretroviral drugs
Orlistat can decrease cyclosporine exposure. Orlistat and cyclosporine should not be simultaneously co-administered
Patients should be strongly encouraged to take a multivitamin supplement that contains fat-soluble vitamins to ensure adequate nutrition because Orlistat has been shown to reduce the absorption of some fat-soluble vitamins and beta-carotene
Rare cases of severe liver injury with hepatocellular necrosis or acute hepatic failure have been reported, with some of these cases resulting in liver transplant or death
Patients may develop increased levels of urinary oxalate following treatment with Orlistat. Monitor renal function in patients at risk for renal insufficiency.
Substantial weight loss can increase the risk of cholelithiasis
Exclude organic causes of obesity (e.g., hypothyr oid ism) before prescribing Orlistat
Patients should be advised to adhere to dietary guidelines. Gastrointestinal events may increase when Orlistat is taken with a diet high in fat (>30% total daily calories from fat)
It is not known if Orlistat is present in human milk. Caution should be exercised when Orlistat is administered to a nursing woman.
The most commonly observed adverse events (incidence of ≥5% and twice that of placebo) were oily spotting, flatus with discharge, fecal urgency, fatty/oily stool, oily evacuation, increased defecation and fecal incontinence. In general, the first occurrence of these events was within 3 months of starting therapy.
Overall, approximately 50% of all episodes of GI adverse events associated with Orlistat treatment lasted for less than 1 week, and a majority lasted for no more than 4 weeks. However, GI adverse events may occur in some individuals over a period of 6 months or longer.
Report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch.
IMPORTANT SAFETY INFORMATION
CONTRAVE can cause serious side effects including:
Suicidal thoughts or actions: One of the ingredients in CONTRAVE is bupropion. Bupropion has caused some people to have suicidal thoughts or actions or unusual changes in behavior, whether or not they are taking medicines used to treat depression. Bupropion may increase the risk of suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment. If you already have depression or other mental illnesses, taking bupropion may cause it to get worse, especially within the first few months of treatment.
While taking CONTRAVE, you or your family members should pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when you start taking CONTRAVE or when your dose changes.
Stop taking CONTRAVE and call a healthcare provider right away if you or your family members notice any of the following symptoms, especially if they are new, worse, or worry you: thoughts about suicide or dying; attempts to commit suicide; depression; anxiety; feeling agitated or restless; panic attacks; irritability; aggression, anger, or violence; acting on dangerous impulses; an extreme increase in activity and talking; other unusual changes in behavior or mood; trouble sleeping.
CONTRAVE is not approved for use in children under the age of 18.
Do not take CONTRAVE if you have uncontrolled high blood pressure; have or have had seizures; use other medicines that contain bupropion such as WELLBUTRIN, WELLBUTRIN SR, WELLBUTRIN XL, APLENZIN and ZYBAN; have or have had an eating disorder called anorexia or bulimia; are dependent on opioid pain medicines or use medicines to help stop taking opioids, or are in opiate withdrawal; drink a lot of alcohol and abruptly stop drinking, or use medicines called sedatives (these make you sleepy), benzodiazepines, or anti‐seizure medicines and stop using them all of a sudden; are taking or have taken medicines called monoamine oxidase inhibitors (MAOIs) in the past 14 days; or are allergic to any of the ingredients in CONTRAVE.
Tell your healthcare provider about all of your medical conditions including if you have: depression or other mental illnesses; attempted suicide; seizures; head injury; tumor or infection of brain or spine; low blood sugar or low sodium; liver or kidney problems; high blood pressure; heart attack, heart problems, or stroke; eating disorder; drinking a lot of alcohol; prescription medicine or street drug abuse; are 65 or older; diabetes; pregnant or planning to become pregnant; or breastfeeding. Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
CONTRAVE may cause serious side effects, including:
Seizures. There is a risk of having a seizure when you take CONTRAVE. The risk of seizure is higher in people who take higher doses of CONTRAVE, have certain medical conditions, or take CONTRAVE with certain other medicines. Do not take any other medicines while you are taking CONTRAVE unless your healthcare provider has said it is okay to take them. If you have a seizure while taking CONTRAVE, stop taking CONTRAVE and call your healthcare provider right away.
Risk of opioid overdose. Do not take large amounts of opioids, including opioid-containing medicines, such as heroin or prescription pain pills, to try to overcome the opioid-blocking effects of naltrexone. This can lead to serious injury, coma, or death.
Get emergency medical help right away if you take opioids and you:
have trouble breathing
become very drowsy with slowed breathing
have slow, shallow breathing
feel faint, very dizzy, confused, or have unusual symptoms
Sudden opioid withdrawal. People who take CONTRAVE must not use any type of opioid including street drugs, prescription pain medicines, cough, cold, or diarrhea medicines that contain opioids, or opioid dependence treatments, for at least 7 to 10 days before starting CONTRAVE. Using opioids in the 7 to 10 days before you start taking CONTRAVE may cause you to suddenly have symptoms of opioid withdrawal when you take it. Sudden opioid withdrawal can be severe, and you may need to go to the hospital. Tell your healthcare provider you are taking CONTRAVE before a medical procedure or surgery.
Severe allergic reactions. Stop taking CONTRAVE and call your healthcare provider or go to the nearest hospital emergency room right away if you have any of the following signs and symptoms of an allergic reaction: rash, itching, hives, fever, swollen lymph glands, painful sores in your mouth or around your eyes, swelling of your lips or tongue, chest pain, or trouble breathing.
Increases in blood pressure or heart rate. Some people may get high blood pressure or have a higher heart rate when taking CONTRAVE. Your healthcare provider should check your blood pressure and heart rate before you start taking, and while you take CONTRAVE.
Liver damage or hepatitis. Stop taking CONTRAVE and tell your healthcare provider if you have any of the following symptoms of liver problems: stomach area pain lasting more than a few days, dark urine, yellowing of the whites of your eyes, or tiredness. Your healthcare provider may need to stop treating you with CONTRAVE if you get signs or symptoms of a serious liver problem.
Manic episodes. Bupropion can cause some people who were manic or depressed in the past to become manic or depressed again.
Visual problems (angle-closure glaucoma). Signs and symptoms may include: eye pain, changes in vision, swelling or redness in or around the eye. Talk with your healthcare provider to find out if you are at risk for angle-closure glaucoma and to get treatment to prevent it if you are at risk.
Increased risk of low blood sugar in people with type 2 diabetes mellitus who also take medicines to treat their diabetes (such as insulin or sulfonylureas). You should check your blood sugar before you start taking CONTRAVE and while you take CONTRAVE.
The most common side effects of CONTRAVE include nausea, constipation, headache, vomiting, dizziness, trouble sleeping, dry mouth, and diarrhea. These are not all of the possible side effects of CONTRAVE.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Metformin is approved by the FDA as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Healthcare providers have the discretion to prescribe a medication for other uses as part of the practice of medicine.
Important Safety Information
Metformin is a prescription medicine that is FDA approved to treat Type 2 diabetes mellitus when hyperglycemia cannot be managed with diet and exercise alone.
When prescribed off-label Metformin may help some adults with overweight or obesity lose weight and keep the weight off.
What is the most important information I should know about Metformin tablets?
Serious side effects can happen in people taking Metformin tablets, including:
Lactic Acidosis. Metformin, the medicine in Metformin tablets, can cause a rare, but serious, side effect called lactic acidosis (a build-up of lactic acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in a hospital.
Stop taking Metformin tablets and call your healthcare provider right away if you get any of the following symptoms of lactic acidosis:
Feel very weak and tired
Have unusual (not normal) muscle pain
Have trouble breathing
Have unusual sleepiness or sleep longer than usual
Have unexplained stomach or intestinal problems with nausea and vomiting, or diarrhea
Feel cold, especially in your arms and legs
Feel dizzy or lightheaded
Have a slow or irregular heartbeat
You have a higher chance of getting lactic acidosis if you:
Have kidney problems. People whose kidneys are not working properly should not take Metformin tablets.
Have liver problems.
Have congestive heart failure that requires treatment with medicines.
Drink a lot of alcohol (very often or short-term "binge" drinking).
Get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids.
Have certain x-ray tests with injectable dyes or contrast agents.
Have surgery.
Have a heart attack, severe infection, or stroke.
Are 80 years of age or older and have not had your kidney function tested.
Do not take Metformin tablets if you:
Have kidney problems
Have an abnormal creatinine level (not to exceed 500mg daily)
Are allergic to the metformin in Metformin tablets or any of the ingredients in Metformin tablets. See the medication guide for the full list of ingredients.
Are going to get an injection of dye or contrast agents for an x-ray procedure or if you are going to have surgery and not able to eat or drink much. In these situations, Metformin tablets will need to be stopped for a short time. Talk to your healthcare provider about when you should stop Metformin tablets and when you should start Metformin tablets again.
What should I tell my healthcare provider before taking Metformin tablets?
Before taking Metformin tablets, tell your healthcare provider if you:
Have type 1 diabetes. Metformin tablets should not be used to treat people with type 1 diabetes.
Have a history or risk for diabetic ketoacidosis (high levels of certain acids, known as ketones, in the blood or urine). Metformin tablets should not be used for the treatment of diabetic ketoacidosis.
Have kidney problems.
Have an abnormal creatinine level
Have liver problems.
Have heart problems, including congestive heart failure.
Are older than 80 years. If you are over 80 years old you should not take Metformin tablets unless your kidneys have been checked and they are normal.
Drink alcohol very often, or drink a lot of alcohol in short-term "binge" drinking.
Are taking insulin.
Have any other medical conditions.
Are pregnant or plan to become pregnant. It is not known if metformin will harm your unborn baby. If you are pregnant, talk with your healthcare provider about the best way to control your blood sugar while you are pregnant.
Are breast-feeding or plan to breast-feed. It is not known if metformin passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby while you take Metformin tablets.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
Metformin tablets may affect the way other medicines work, and other medicines may affect how Metformin tablets work.
Common side effects of Metformin tablets include diarrhea, nausea, and upset stomach. These side effects generally go away after you take the medicine for a while. Taking your medicine with meals can help reduce these side effects. Tell your healthcare provider if the side effects bother you a lot, last for more than a few weeks, come back after they've gone away, or start later in therapy. You may need a lower dose or need to stop taking the medicine for a short period or for good.
About 3 out of every 100 people who take Metformin tablets have an unpleasant metallic taste when they start taking the medicine. It lasts for a short time.
Metformin tablets rarely cause hypoglycemia (low blood sugar) by themselves. However, hypoglycemia can happen if you do not eat enough, if you drink alcohol, or if you take other medicines to lower blood sugar.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
The above health information is provided for educational purposes only and is not intended to replace discussions with a healthcare provider. All decisions regarding patient care must be made with a healthcare provider, considering the unique characteristics of the patient. The product information provided is intended only for residents of the United States. The products discussed herein may have different product labeling in different countries.
Warning
○ Drugs like this one have raised the chance of suicidal thoughts or actions in children and young adults. The risk may be greater in people who have had these thoughts or actions in the past. All people who take this drug need to be watched closely. Call the doctor right away if signs like depression, nervousness, restlessness, grouchiness, panic attacks, or changes in mood or actions are new or worse. Call the doctor right away if any thoughts or actions of suicide occur.
What is this drug used for?
What do I need to tell my doctor BEFORE I take this drug?
This is not a list of all drugs or health problems that interact with this drug.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take this drug with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take this drug?
For all patients taking this drug:
You will need to talk about the benefits and risks to you and the baby.
If you smoke:
What are some side effects that I need to call my doctor about right away? WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
What are some other side effects of this drug?
All drugs may cause side effects. However , many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
All products:
Extended-release tablets:
How is this drug best taken?
Use this drug as ordered by your doctor. Read all information given to you. Follow all instructions closely.
For all uses of this drug:
What do I do if I miss a dose?
How do I store and/or throw out this drug?
General drug facts
Topiramate is FDA approved
to treat certain types of seizures (partial-onset seizures and primary generalized tonic-clonic seizures) in adults and children 2 years and older.
with other medicines to treat certain types of seizures (partial-onset seizures, primary generalized tonic-clonic seizures, and seizures associated with Lennox-Gastaut syndrome) in adults and children 2 years and older.
to prevent migraine headaches in adults and adolescents 12 years and older.
Topiramate is prescribed off-label and may help some adults with overweight and obesity lose weight and keep the weight off.
Important Safety Information
What is the most important information I should know about Topiramate?
Topiramate may cause eye problems. Serious eye problems include:
Any sudden decrease in vision with or without eye pain and redness.
A blockage of fluid in the eye causing increased pressure in the eye (secondary angle closure glaucoma).
These eye problems can lead to permanent loss of vision if not treated.
You should call your healthcare provider right away if you have any new eye symptoms, including any new problems with your vision.
Topiramate may cause decreased sweating and increased body temperature (fever). People, especially children, should be watched for signs of decreased sweating and fever, especially in hot temperatures. Some people may need to be hospitalized for this condition. If a high fever, a fever that does not go away, or decreased sweating develops, call your healthcare provider right away.
Topiramate can increase the level of acid in your blood (metabolic acidosis). If left untreated, metabolic acidosis can cause brittle or soft bones (osteoporosis, osteomalacia, osteopenia), kidney stones, can slow the rate of growth in children, and may possibly harm your baby if you are pregnant. Metabolic acidosis can happen with or without symptoms. Sometimes people with metabolic acidosis will:
Feel tired
Not feel hungry (loss of appetite)
Feel changes in heartbeat
Have trouble thinking clearly
Your healthcare provider should do a blood test to measure the level of acid in your blood before and during your treatment with Topiramate. If you are pregnant, you should talk to your healthcare provider about whether you have metabolic acidosis.
Like other antiepileptic drugs, Topiramate may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
Thoughts about suicide or dying
Attempts to commit suicide
New or worse depression
New or worse anxiety
Panic attacks
An extreme increase in activity and talking (mania)
Feeling agitated or restless
Trouble sleeping (insomnia)
New or worse irritability
Acting aggressive, being angry or violent
Acting on dangerous impulses
Other unusual changes in behavior or mood
Topiramate may lower bone mineral density. TOPIRAMATE may decrease the density of bones when used over a long period.
Topiramate may have negative effects on growth in children TOPIRAMATE may slow height increases and weight gain in children and adolescents when used over a long period.
Do not stop Topiramate without first talking to a healthcare provider.
Stopping Topiramate suddenly can cause serious problems.
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
If you have epilepsy and you stop taking Topiramate suddenly, you may have seizures that do not stop. Your healthcare provider will tell you how to stop taking Topiramate slowly.
If you miss a single dose of Topiramate, take it as soon as you can. However, if you are within 6 hours of taking your next scheduled dose, wait until then to take your usual dose of Topiramate and skip the missed dose. Do not double your dose. If you have missed more than one dose, you should call your healthcare provider for advice.
How can I watch for early symptoms of suicidal thoughts and actions?
Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Topiramate can harm your unborn baby.
If you take Topiramate during pregnancy, your baby has a higher risk for birth defects called cleft lip and cleft palate. These defects can begin early in pregnancy, even before you know you are pregnant.
Cleft lip and cleft palate may happen even in children born to women who are not taking any medicines and do not have other risk factors.
There may be other medicines to treat your condition that have a lower chance of birth defects.
All women of childbearing age should talk to their healthcare providers about using other possible treatments instead of Topiramate. If the decision is made to use Topiramate, you should use effective birth control (contraception) unless you are planning to become pregnant. You should talk to your doctor about the best kind of birth control to use while you are taking Topiramate.
Tell your healthcare provider right away if you become pregnant while taking Topiramate. You and your healthcare provider should decide if you will continue to take Topiramate while you are pregnant.
If you take Topiramate during pregnancy, your baby may be smaller than expected at birth. The long-term effects of this are not known. Talk to your healthcare provider if you have questions about this risk during pregnancy.
Metabolic acidosis may have harmful effects on your baby. Talk to your healthcare provider if Topiramate has caused metabolic acidosis during your pregnancy.
Pregnancy Registry: If you become pregnant while taking Topiramate, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of Topiramate and other antiepileptic drugs during pregnancy.
Before taking Topiramate, tell your healthcare provider about all of your medical conditions, including if you:
Have or have had depression, mood problems, or suicidal thoughts or behavior
Have kidney problems, have kidney stones, or are getting kidney dialysis
Have a history of metabolic acidosis (too much acid in the blood)
Have liver problems
Have weak, brittle, or soft bones (osteomalacia, osteoporosis, osteopenia, or decreased bone density)
Have lung or breathing problems
Have eye problems, especially glaucoma
Have diarrhea
Have a growth problem
Are on a diet high in fat and low in carbohydrates, which is called a ketogenic diet
Are having surgery
Are pregnant or plan to become pregnant
Are breastfeeding or plan to breastfeed? Topiramate passes into breast milk. Breastfed babies may be sleepy or have diarrhea. It is not known if the Topiramate that passes into breast milk can cause serious harm to your baby. Talk to your healthcare provider about the best way to feed your baby if you take Topiramate.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Topiramate and other medicines may affect each other causing side effects. Especially tell your healthcare provider if you take:
Valproic acid (such as Depakene® or Depakote®).
Any medicines that impair or decrease your thinking, concentration, or muscle coordination.
Birth control that contains hormones (such as pills, implants, patches or injections). Topiramate may make your birth control less effective. Tell your healthcare provider if your menstrual bleeding changes while you are using birth control and Topiramate.
Ask your healthcare provider if you are not sure if your medicine is listed above. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine. Do not start a new medicine without talking with your healthcare provider.
What are the possible side effects of Topiramate?
Topiramate may cause serious side effects including:
See “What is the most important information I should know about Topiramate?”
High blood ammonia levels. High ammonia in the blood can affect your mental activities, slow your alertness, make you feel tired, or cause vomiting. This has happened when Topiramate is taken with a medicine called valproic acid (Depakene® or Depakote®).
Effects on thinking and alertness. Topiramate may affect how you think and cause confusion, problems with concentration, attention, memory, or speech. TOPIRAMATE may cause depression or mood problems, tiredness, and sleepiness.
Dizziness or loss of muscle coordination.
Serious skin reactions. Topiramate may cause a severe rash with blisters and peeling skin, especially around the mouth, nose, eyes, and genitals (Stevens-Johnson syndrome). Topiramate may also cause a rash with blisters and peeling skin over much of the body that may cause death (toxic epidermal necrolysis). Call your healthcare provider right away if you develop a skin rash or blisters.
Kidney stones. Drink plenty of fluids when taking Topiramate to decrease your chances of getting kidney stones.
Low body temperature. Taking Topiramate when you are also taking valproic acid can cause a drop in body temperature to less than 95°F, feeling tired, confusion, or coma.
Call your healthcare provider right away if you have any of the symptoms above.
The most common side effects of Topiramate include:
Tingling of the arms and legs (paresthesia)
Not feeling hungry
Nausea
A change in the way foods taste
Diarrhea
Weight loss
Nervousness
Upper respiratory tract infection
Speech problems
Tiredness
Dizziness
Sleepiness/drowsiness
Slow reactions
Difficulty with memory
Pain in the abdomen
Fever
Abnormal vision
Decreased feeling or sensitivity, especially in the skin
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
The above health information is provided for educational purposes only and is not intended to replace discussions with a healthcare provider. All decisions regarding patient care must be made with a healthcare provider, considering the unique characteristics of the patient. The product information provided is intended only for residents of the United States. The products discussed herein may have different product labeling in different countries.